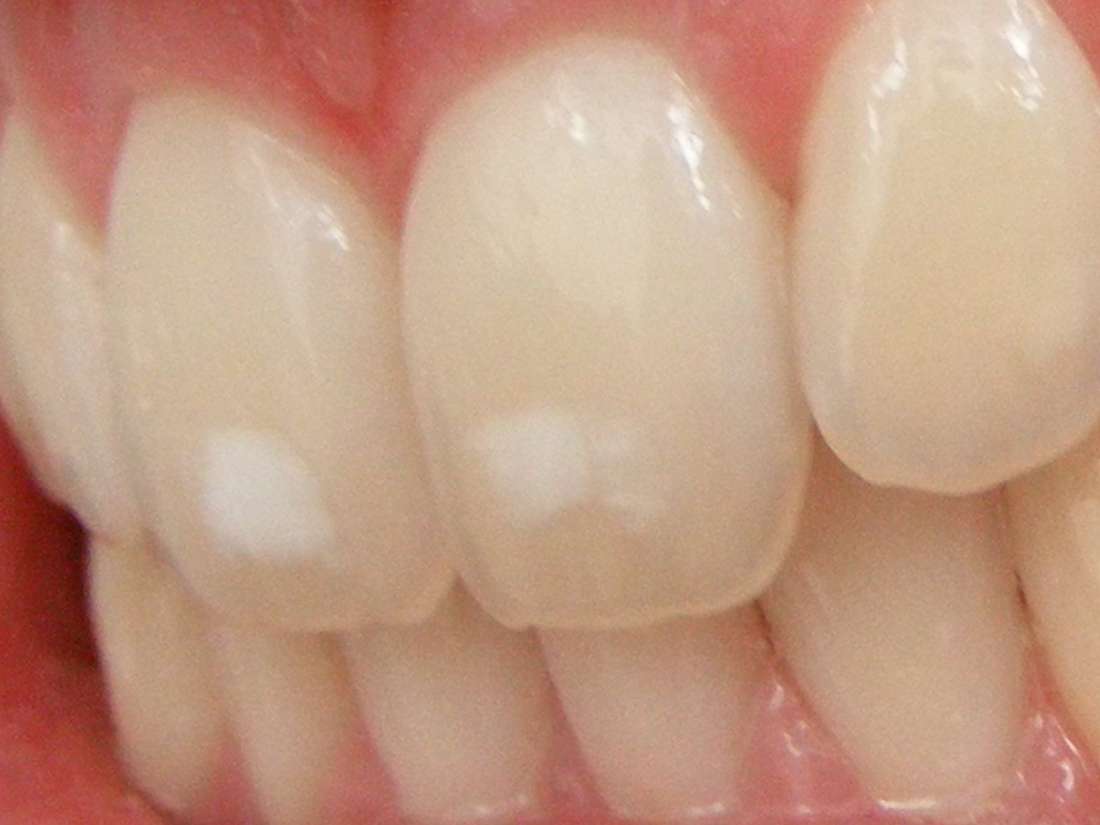
Exposure to high concentrations of fluoride during childhood, when teeth are developing, can result in mild dental fluorosis. There will be tiny white streaks or specks in the enamel of the tooth. This does not affect the health of the teeth, but the discoloration may be noticeable. Breastfeeding infants or making up formula milk with fluoride-free water can help protect small children from fluorosis. Children below the age of 6 years should not use a mouthwash that contains fluoride. Children should be supervised when brushing their teeth to ensure they do not swallow toothpaste. Skeletal fluorosisExcess exposure to fluoride can lead to a bone disease known as skeletal fluorosis. Over many years, this can result in pain and damage to bones and joints. The bones may become hardened and less elastic, increasing the risk of fractures. If the bones thicken and bone tissue accumulates, this can contribute to impaired joint mobility. Thyroid problemsIn some cases, excess fluoride can damage the parathyroid gland. This can result in hyperparathyroidism, which involves uncontrolled secretion of parathyroid hormones. This can result in a depletion of calcium in bone structures and higher-than-normal concentrations of calcium in the blood. Lower calcium concentrations in bones make them more susceptible to fractures. Neurological problemsIn 2017, a report was published suggesting that exposure to fluoride before birth could lead to poorer cognitive outcomes in the future. The researchers measured fluoride levels in 299 women during pregnancy and in their children between the ages of 6 and 12 years. They tested cognitive ability at the ages of 4 years and between 6 and 12 years. Higher levels of fluoride were associated with lower scores on IQ tests. In 2014, fluoride was documented as a neurotoxin that could be hazardous to child development, along with 10 other industrial chemicals, including lead, arsenic, toluene, and methylmercury. RELATED ARTICLE
Other health problemsAccording to the International Association of Oral Medicine and Toxicology (IAOMT), an organization that campaigns against the use of added fluoride, it may also contribute to the following health problems:
One review describes fluoride as an "extreme electron scavenger" with an "insatiable appetite for calcium." The researchers call for the balance of risks and benefits to be reconsidered. Fluoride poisoningAcute, high-level exposure to fluoride can lead to:
This will not result from drinking tap water. It is only likely to happen in cases of accidental contamination of drinking water, due, for example to an industrial fire or explosion. It is worth remembering that many substances are harmful in large quantities but helpful in small amounts. UsesFlouride exists in many water supplies, and it is added to drinking water in many countries.
It is also used in the following dental products:
Non-dental sources of flouride include:
Excess fluoride exposure may come from:
Not all fluoride exposure is due to adding the chemical to water and dental products. Some geographical areas have drinking water that is naturally high in fluoride, for example, southern Asia, the eastern Mediterranean, and Africa. Side effectsPossible side effects of excessive fluoride intake include:
Other possible side effects are listed under the "risks" section above. BenefitsThe American Dental Association (ADA) says fluoride in water benefits communities because it:
Fluoride is present in natural water. Adding fluoride, says the ADA, is like fortifying milk with vitamin D, orange juice with calcium, or cereals with B vitamins and folic acid. Studies continue to show that adding fluoride to water supports dental health. A Cochrane review published in 2015 found that when fluoride was introduced to water:
Applying fluoride on children's teeth can prevent or slow decay. How does it work?Fluoride prevents tooth decay by:
This involves the following processes: Protection from demineralization: When bacteria in the mouth combine with sugars, they produce acid. This acid can erode tooth enamel and damage our teeth. Fluoride can protect teeth from demineralization that is caused by the acid. Remineralization: If acid has already caused some damage to the teeth, fluoride accumulates in the demineralized areas and begins strengthening the enamel. This is remineralization. Who benefits the most?Everyone can benefit from added dental protection, but those who can benefit particularly are people who:
Most public health authorities and medical associations worldwide recommend that children and adults receive some fluoride, to protect their teeth from decay. FactsHere are some facts supporting the use of fluoride:
Here are some arguments against its use, from the IAOMT:
A range of fluoride and fluoride-free dental products are available for purchase onlline. ControversyThe controversy continues over whether it is a good idea to add fluoride to water or not. In 2000, German researchers reported that tooth decay fell in cities where fluoride ceased to be added to the water. However, they called for further investigation into the reasons for this decline, which they said could be due to improved attitudes toward dental health and easier access to dental health products, compared with the years before fluoride was added. They suggested that their findings might support the argument that caries can continue to fall if the concentration of fluoride is reduced from 1 part per million (ppm) to below 0.2 ppm. How much fluoride is recommended?The Department of Health and Human Services (DHHS) sets the optimal level of fluoride for preventing tooth decay at 0.7 ppm, or 0.7 milligrams (mg) in every liter of water. The previous figure, in force from 1962 to 2015, was 0.7 to 1.2 ppm. In 2015, it was revised to the lower limit. The aim of this optimal level is to promote public health. What does the WHO say?The World Health Organization (WHO) notes that long-term exposure to drinking water that contains more than 1.5 ppm fluoride can lead to health problems. The WHO's guideline limit is 1.5 ppm. How much does the EPA allow?The Environmental Protection Agency (EPA) aims to protect people from over-exposure to toxic chemicals. It sets the maximum allowable level at 4 ppm, and a secondary maximum level at 2 ppm. People are asked to inform the EPA if levels are above 2 ppm. Levels above 4 ppm could be hazardous. In areas where water naturally contains higher levels of fluoride, community water systems must ensure that the maximum level is no higher than 4 ppm. TakeawayAs with any substance, excess intake or exposure can be harmful. It is important not to use any fluoride supplements without first speaking to a dentist.
|
1-3.
|
 Possible benefits of drinking water range from keeping the kidneys healthy to losing weight. |
To function properly, all the cells and organs of the body need water.
Here are some reasons our body needs water:
1. It lubricates the joints
Cartilage, found in joints and the disks of the spine, contains around 80 percent water. Long-term dehydration can reduce the joints' shock-absorbing ability, leading to joint pain.
2. It forms saliva and mucus
Saliva helps us digest our food and keeps the mouth, nose, and eyes moist. This prevents friction and damage. Drinking water also keeps the mouth clean. Consumed instead of sweetened beverages, it can also reduce tooth decay.
3. It delivers oxygen throughout the body
Blood is more than 90 percent water, and blood carries oxygen to different parts of the body.
4. It boosts skin health and beauty
With dehydration, the skin can become more vulnerable to skin disorders and premature wrinkling.
5. It cushions the brain, spinal cord, and other sensitive tissues
Dehydration can affect brain structure and function. It is also involved in the production of hormones and neurotransmitters. Prolonged dehydration can lead to problems with thinking and reasoning.
6. It regulates body temperature
Water that is stored in the middle layers of the skin comes to the skin's surface as sweat when the body heats up. As it evaporates, it cools the body. In sport.
Some scientists have suggested that when there is too little water in the body, heat storage increases and the individual is less able to tolerate heat strain.
Having a lot of water in the body may reduce physical strain if heat stress occurs during exercise. However, more research is needed into these effects.
7, The digestive system depends on it
The bowel needs water to work properly. Dehydration can lead to digestive problems, constipation, and an overly acidic stomach. This increases the risk of heartburn and stomach ulcers.
8. It flushes body waste
Water is needed in the processes of sweating and removal of urine and feces.
9. It helps maintain blood pressure
A lack of water can cause blood to become thicker, increasing blood pressure.
10. The airways need it
When dehydrated, airways are restricted by the body in an effort to minimize water loss. This can make asthma and allergies worse.
11. It makes minerals and nutrients accessible
These dissolve in water, which makes it possible for them to reach different parts of the body.
12. It prevents kidney damage
The kidneys regulate fluid in the body. Insufficient water can lead to kidney stones and other problems.
13. It boosts performance during exercise
 Dehydration during exercise may hinder performance. |
Some scientists have proposed that consuming more water might enhance performance during strenuous activity.
More research is needed to confirm this, but one review found that dehydration reduces performance in activities lasting longer than 30 minutes.
14. Weight loss
Water may also help with weight loss, if it is consumed instead of sweetened juices and sodas. "Preloading" with water before meals can help prevent overeating by creating a sense of fullness.
15. It reduces the chance of a hangover
When partying, unsweetened soda water with ice and lemon alternated with alcoholic drinks can help prevent overconsumption of alcohol.
Kidney damage
Water helps dissolve minerals and nutrients, making them more accessible to the body. It also helps remove waste products.
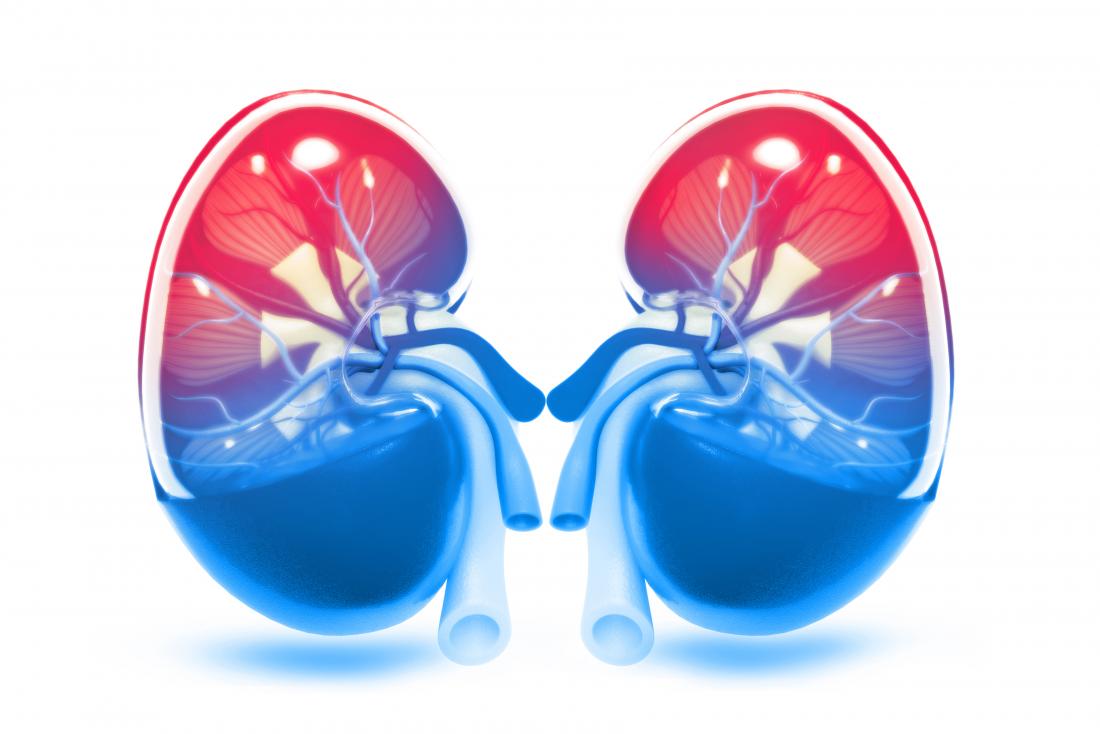 The kidneys play a key role in balancing fluid levels. |
These two functions make water vital to the kidneys.
Every day, the kidneys filter around 120-150 quarts of fluid.
Of these, approximately 1-2 quarts are removed from the body in the form of urine, and the rest is recovered by the bloodstream.
Water is essential for the kidneys to function.
If the kidneys do not function properly, waste products and excess fluid can build up inside the body.
Untreated, chronic kidney disease can lead to kidney failure. The organs stop working, and either dialysis or kidney transplantation is required.
Urinary tract infections (UTIs) are the second most common type of infection in the body. They account for around 8.1 million visits to health care providers in the U.S. every year.
If infections spread to the upper urinary tract, including the kidneys, permanent damage can result. Sudden, or acute, kidney infections can be life-threatening, particularly if septicemia occurs.
Drinking plenty of water is a simple way to reduce the risk of developing a UTI and to help treat an existing UTI.
Kidney stones interfere with how the kidneys work. When present, can complicate UTIs. These complicated UTIs tend to require longer periods of antibiotics to treat them, typically lasting 7 to 14 days.
The leading cause of kidney stones is a lack of water. People who report them often do not drink the recommended daily amount of water. Kidney stones may also increase the risk of chronic kidney disease.
In November 2014, the American College of Physicians issued new guidelines for people who have previously developed kidney stones. The guidelines state that increasing fluid intake to enable 2 liters of urination a day could decrease the risk of stone recurrence by at least half with no side effects.
Dehydration happens if we use and lose more water than the body takes in. It can lead to an imbalance in the body's electrolytes. Electrolytes, such as potassium, phosphate, and sodium, help carry electrical signals between cells. The kidneys keep the levels of electrolytes in the body stable when they function properly.
When the kidneys are unable to maintain a balance in the levels of electrolytes, these electrical signals become mixed up. This can lead to seizures, involving involuntary muscle movements and loss of consciousness.
In severe cases, dehydration can lead to kidney failure, which can be life-threatening. Possible complications of chronic kidney failure include anemia, damage to the central nervous system, heart failure, and a compromised immune system.
RELATED ARTICLE
 | Is carbonated water bad for you? Should I drink soda water? Find out more READ NOW |
Sources
Some of the water required by the body is obtained through foods with a high water content, such as soups, tomatoes, oranges, but most come through drinking water and other beverages.
During everyday functioning, water is lost by the body, and this needs to be replaced. We notice that we lose water through activities such as sweating and urination, but water is lost even when breathing.
Drinking water, whether from the tap or a bottle, is the best source of fluid for the body.
Milk and juices are also good sources of fluid, but beverages containing alcohol and caffeine, such as soft drinks, coffee, and beer, are not ideal because they often contain empty calories. Drinking water instead of soda can help with weight loss.
It was previously thought that caffeinated beverages had diuretic properties, meaning that they cause the body to release water. However, studies show that fluid loss because of caffeinated drinks is minimal.
Recommended intake
 How much water we need to consume is influenced by the climate. |
The amount of water needed each day varies from person to person, depending on how active they are, how much they sweat, and so on.
There is no fixed amount of water that must be consumed daily, but there is general agreement on what a healthy fluid intake is.
According to the U.S. National Academies of Sciences, Engineering, and Medicine, the average recommended daily intake of water from both food and drink is:
- For men: Around 3.7 liters or 125 ounces
- For women: Around 2.7 liters or 91 ounces
This would be around 15.5 cups for men and just over 11 cups for women. However, around 80 percent of this should come from drinks, including water, and the rest will be from food.
This means that:
- Men should drink around 100 ounces, or 12.5 cups of fluid
- Women should drink around 73 ounces, or just over 9 cups
Fresh fruits and vegetables and all non-alcoholic fluids count towards this recommendation.
Times when it is most important to drink plenty of water include:
- when you have a fever
- when the weather is hot
- if you have diarrhea and vomiting
- when you sweat a lot, for example, due to physical activity
Facts
Here are some facts about water:
- Babies and children have a higher percentage of water than adults. When babies are born, they are about 78 percent water, but this falls to 65 percent by the age of 1 year.
- Fatty tissue has less water than lean tissue.
- Men have more water than women, as a percentage.
Do we drink enough water?
A study carried out by the Centers for Disease Control and Prevention (CDC) in 2013 analyzed data from the National Cancer Institute's 2007 Food Attitudes and Behaviors Survey.
Out of a sample of 3,397 adults, the researchers found:
- 7 percent of adults reported no daily consumption of drinking water
- 36 percent of adults reported drinking 1-3 cups of drinking water a day
- 35 percent of adults reported drinking 4-7 cups of drinking water a day
- 22 percent of adults reported drinking 8 cups or more a day
People were more likely to drink less than 4 cups of drinking water daily if they consumed 1 cup or less of fruits or vegetables a day.
The study only measured the intake of drinking water. Fluid can be gained from other beverages, but water is best because it is calorie-free, caffeine-free, and alcohol-free.
Seven percent of respondents reported drinking no water at all daily, and those who drank a low volume of water also consumed less fruit and vegetables. This suggests that a certain number of people are risking their health by not getting enough fluid.
Even if the respondents reporting low levels of water intake were obtaining enough fluid, it is likely that they would be obtaining it from sources that could potentially compromise their health in other ways.
"The biologic requirement for water may be met with plain water or via foods and other beverages," write the study authors. "Results from previous epidemiologic studies indicate that water intake may be inversely related to volume of calorically sweetened beverages and other fluid intake."
1-4.
|
 Dr. Broadbent suggests that studies finding an association between water fluoridation and reduced IQ tend to have used poor research methodology with a high risk of bias. |
The IQ scores for 992 participants were examined between the ages of 7-13. Of these people, 942 were tested again at age 38. The scores of tests assessing verbal comprehension, perceptual reasoning, working memory and processing speed were also available to the Otago researchers.
The team controlled the results for factors that are known to influence IQ variation in childhood, such as the socioeconomic status of the parents, birth weight and breastfeeding, as well as achievement in secondary and tertiary education, which are thought to influence adult IQ.
Lead author Dr. Jonathan Broadbent describes the team's findings:
"Our analysis showed no significant differences in IQ by fluoride exposure, even before controlling for the other factors that might influence scores. In line with other studies, we found breastfeeding was associated with higher child IQ, and this was regardless of whether children grew up in fluoridated or non-fluoridated areas."
Dr. Broadbent suggests that studies finding an association between water fluoridation and reduced IQ tend to have used poor research methodology with a high risk of bias. Speaking to Medical News Today, he said of the Harvard study: "The authors stated that each of the articles reviewed had deficiencies, in some cases rather serious. It is a meta-analysis based on poor quality research."
He adds that the Dunedin Multidisciplinary Study, by comparison, is world-renowned for the quality of its data and rigor of its analysis.
In conclusion, Dr. Broadbent says:
"Our findings will hopefully help to put another nail in the coffin of the complete canard that fluoridating water is somehow harmful to children's development. In reality, the total opposite is true, as it helps reduce the tooth decay blighting the childhood of far too many New Zealanders."
RELATED COVERAGE
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||